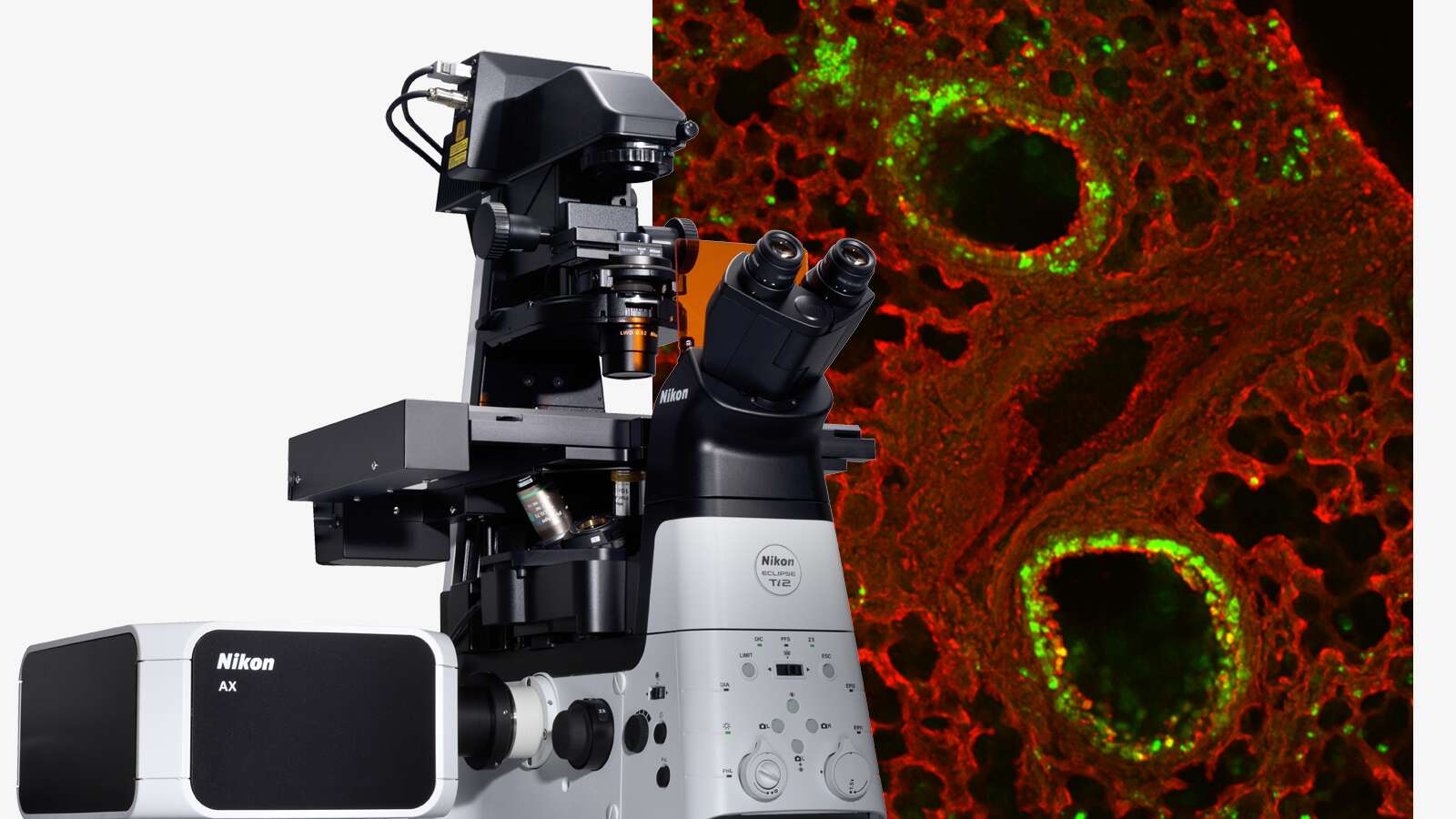
Focus On Virology
Due to the extremely small size of viruses (5-300 nm), their structure and function have always been a challenge to study. Over recent centuries, many viruses have been discovered, and vaccines against them developed, but it was the invention of the electron microscope in 1931 that enabled their complex structures to be visualized. Since then, Nikon has become an expert in providing advanced light microscopy systems to image virus structure and infectivity in real time with high resolution and high throughput. In times when rapid and reliable analysis of viruses is essential, the importance of such equipment has become even more apparent.
Image courtesy of Rudolph Reimer, Heinrich Pette Institute, Leibniz Institute for Experimental Virology