News
Neuroscientist and Founder of the Nikon Microscopy Center of Excellence in Budapest Featured in “Tech Heroes” Campaign on CNN.com
Nov 19, 2014
Dr. Istvan Katona is the Head of the Department of Molecular and Developmental Neuroscience at the Institute of Experimental Medicine of the Hungarian Academy of Sciences
The Tech Heroes Campaign on CNN.com looks at the work of some of the world's leading researchers and innovators. It explores the challenges and dedication required to push back technological boundaries to create a better future.
The latest "Tech Hero" installment features neuroscientist Dr. Istvan Katona, who has made it his mission in life to further our knowledge of how the brain works, specifically how cells send messages to each other. Dr. Katona is the head of the Department of Molecular and Developmental Neuroscience at the Institute of Experimental Medicine of the Hungarian Academy of Sciences and founded the Nikon Microscopy Center of Excellence at IEM.
Dr. Katona’s work is especially relevant in light of an intensified worldwide focus on mapping the activity of every neuron in the human brain and the recently awarded Nobel Prize in Chemistry to Drs. Eric Betzig, Stefan Hell and William Moerner for their roles in the development of super-resolution microscopy.
We sat down with Dr. Katona, and his Ph.D. mentor Dr. Tamas Freund to discuss to discuss their work, and the mission of the Institute.
Please tell us about your research.
"The mission of our research institute is to carry out cutting-edge discovery research, which will reveal fundamental principles of physiological and pathophysiological operation of the nervous system. We integrate research in the field of molecular and cellular neuroscience into a functional context at the circuit and systems levels, and then test the translational implications of our findings in different models of brain disorders. My research group is primarily focusing on the normal and pathological (epileptic, ischemic) activity of cortical networks, with particular attention to the generation of behaviour-dependent population discharge patterns (theta and gamma oscillations, hippocampal sharp waves). Anatomical, in vitro, in vivo electrophysiological, pharmacological and molecular techniques are combined to elucidate the functional roles of inhibitory cell types in the control of population synchrony and synaptic plasticity in the hippocampus, their local and subcortical modulation via selective afferent pathways (GABAergic and cholinergic septal, as well as serotonergic raphe input) and pre- or postsynaptic receptors. This approach has led to several breakthrough achievements like the discovery of the compartmentalization of endocannabinoid signaling at the nanoscale level and its cell type-specific contribution to neurological disorders (culminated in Nature Medicine, 2008, 14:923-30) or the discovery of a new fast synaptic regulatory mechanism of cortical activity by subcortical neuromodulatory systems (Science, 2009, 326:449-53)."

What applications do you use Nikon microscope systems for?
"Our institute established a special collaboration with Nikon in 2010. In the framework of this collaboration, our researchers use four different setups in a dedicated imaging facility. An A1R confocal microscope primarily serves investigations at the molecular and cellular levels together with an N-STORM system equipped with a confocal, which is used to correlate morphological and molecular properties of identified subcellular compartments of neurons in a quantitative manner. The third setup combines patch-clamp electrophysiology with confocal imaging to correlate presynaptic and postsynaptic physiological signals. The fourth setup is a spinning disc system in combination with patch-clamp setup to correlate synaptic events with population activity. It is difficult to select which applications are the most important, because more than 60 of our researchers used these systems in about 75 projects within the last two years. But definitely our new N-STORM super resolution microscope will open new horizons for several of our research projects."
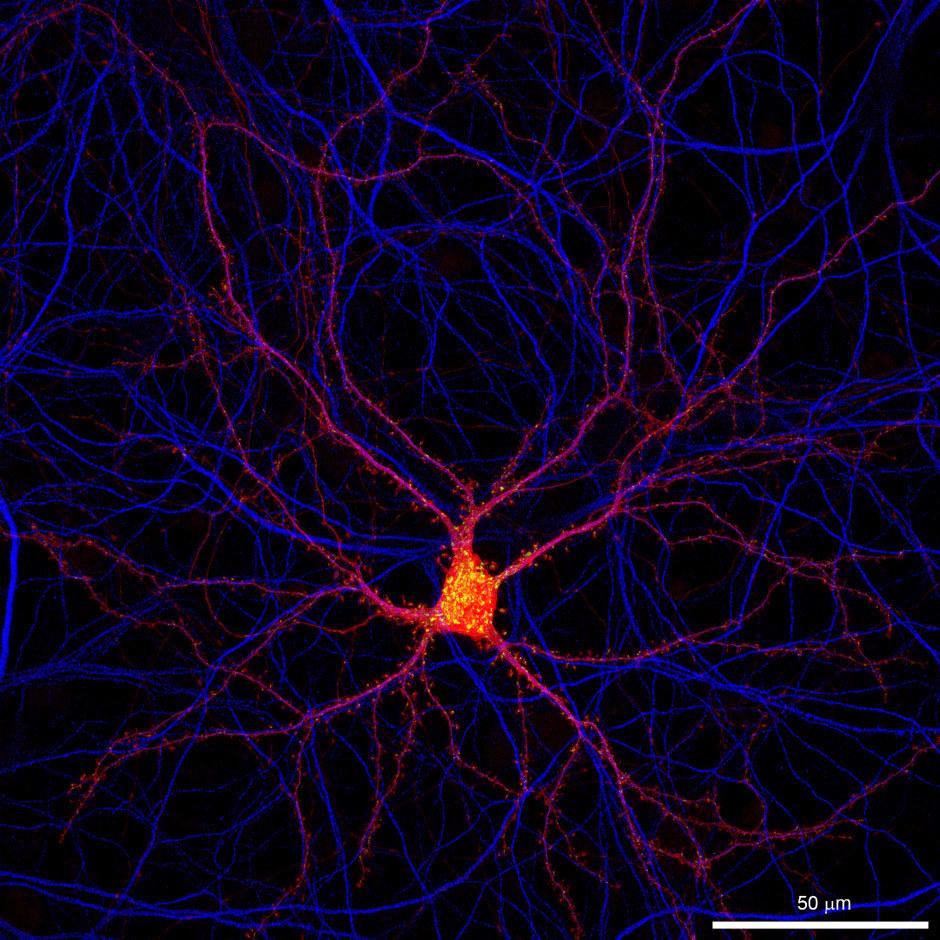
What was the deciding factor for choosing Nikon N-STORM super resolution microscope?
"Overall, we had two important reasons for choosing Nikon for this collaboration. First of all, Nikon is a world leader in the development of cutting-edge microscopy techniques and this special collaboration lets us test their new systems for neuroscience applications soon after their announcement. The N-STORM system is a prime example. The second reason was the outstanding local 24/7 support of Nikon. Optimal calibration and everyday maintenance is a must when many people are using the same system for several distinct applications and the systems are in constant demand."
What is it about the Nikon N-STORM system that you now value after actually using them?
"We are very much impressed by the performance of the new N-STORM system. It provides unprecedented spatial resolution for a fluorescent microscope, and the multicolor feature with its high throughput makes this technology superior to previous microscopic approaches, such as electron microscopy.
Although we have had this system with all the required additional equipment like a high power imaging laser for less than a year, already five research groups are using it routinely for their projects. The first findings have already been published and several others are in the manuscript phase."
The STORM technique is ideal to study molecules at synapses in the brain.
The STORM technique is ideal to study molecules at synapses in the brain.
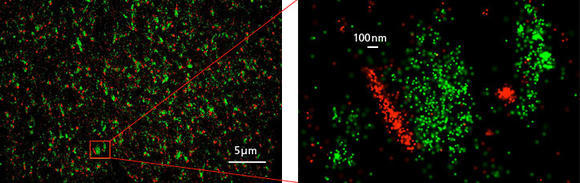
The chemical synapse is the main information channel for nerve cell communication. To accomplish this fundamental function, precisely orchestrated activity of several hundreds, if not thousands of different proteins is required in a tiny compartment of a size of about 1 micron. Before the inventions of super resolution microscopy approaches, it was tremendously difficult to study these molecular assemblies efficiently and to address scientific questions about their changes underlying physiological or pathophysiological activity in the brain.
The N-STORM super resolution microscopy approach enables the combined visualization of multiple synaptic proteins with an unprecedented spatial localization precision. In this first image, hundreds of excitatory synapses are visualized by immunostaining for the type 1 vesicular glutamate transporter (green color), which is a presynaptic protein responsible for filling up synaptic vesicles with the excitatory neurotransmitter, glutamate. The second image depicts that another synaptic protein called Homer (red), which organizes glutamate receptors on the postsynaptic side. Note that the N-STORM molecular imaging of the two proteins clearly distinguishes their compartmentalized distributions at the opposite sites of the chemical synapse.
Excess activity of glutamatergic neurotransmission and increased glutamate levels underly numerous brain disorders like epileptic activity. However, quantitative information about the molecular changes happening after epileptic seizures at chemical synapses was limited. The N-STORM approach now offers an unprecedented opportunity to study change of abundance and position of different proteins perturbed during pathophysiological brain activity.
What is it about the Nikon N-STORM system that you now value after actually using them?
"It is hard to choose, because there are many exciting new developments out in the field now. For the STORM approach, the most important new development will be the possibility of much faster imaging, particularly of live cells. Therefore, we are particularly interested in future N-STORM developments to see how they can be used for physiological experiments in neuroscience."